About Course
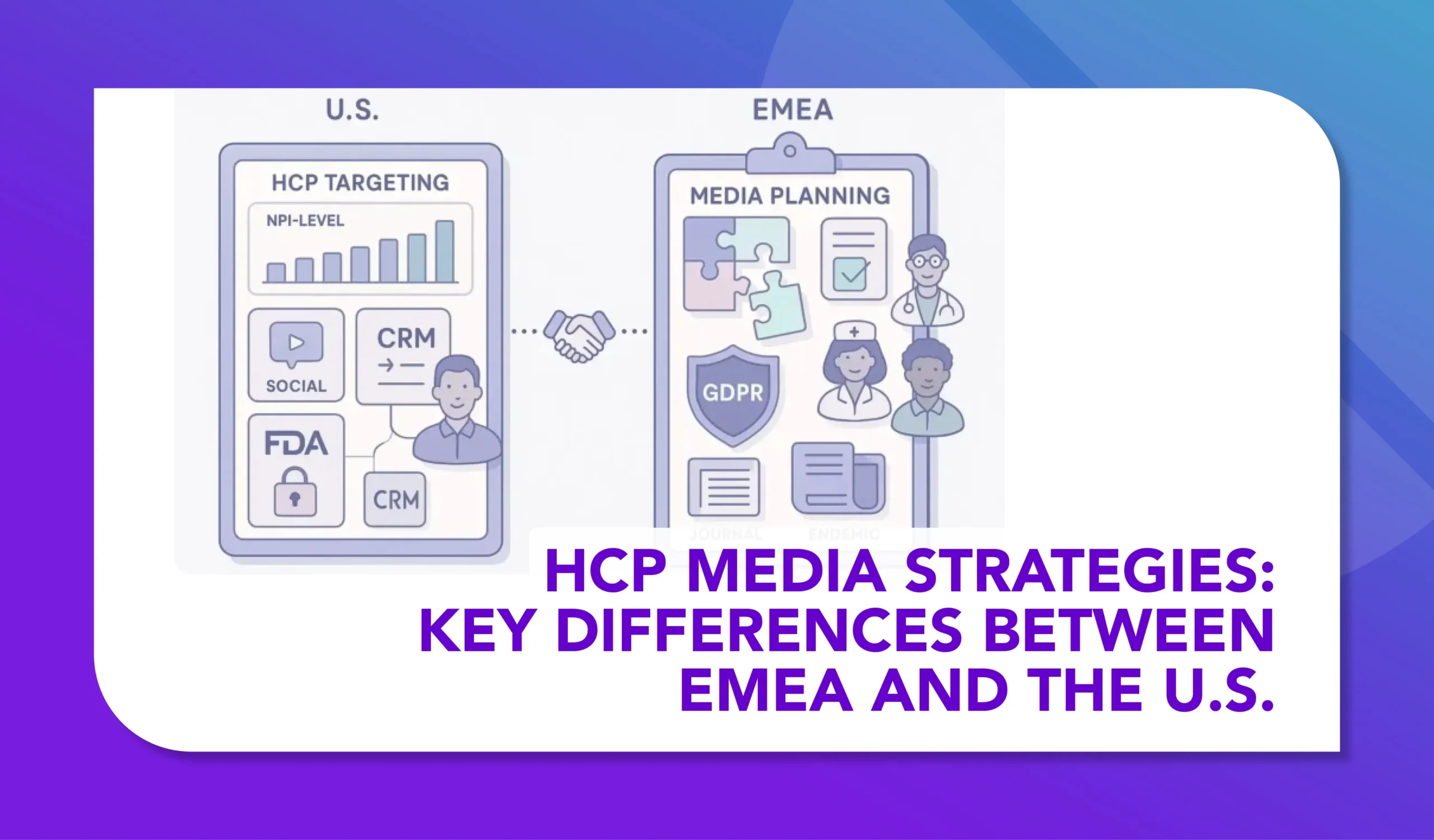
This course explores how HCP media strategies differ between the U.S. and EMEA. From regulatory frameworks to channel choices, tactics, and data use, participants will learn how regional nuances shape what’s possible—and how to adapt campaigns for compliance and impact. The result is a clear framework for building effective strategies that balance global goals with local realities.
Course Content
Navigating the Rules: HCP Media Compliance
-
LESSON: Navigating the Rules: HCP Media Compliance
03:45 -
QUIZ: Navigating the Rules: HCP Media Compliance
Channel Strategy: One Size Doesn’t Fit All
Data, Targeting, and Performance Tracking
SIGN UP FOR FREE TO ACCESS THIS TRAINING
Thank you for diving into solli content!
Sign up for FREE now to complete this training and get unlimited access to all FREE news, resources and training across solli.
Or, upgrade to PREMIUM for unlimited access to all PREMIUM content across solli.